5 - Immobilization
5.1 Direct coupling
During direct coupling the surface sample is linked covalently via carboxyl groups on the sensor chip surface. There are three different covalent derivatizations.
5.1.1 Aldehyde groups (-CHO)
Aldehyde groups (-CHO) will be used to covalently attach the surface sample to the sensor surface. After the activation of the surface using EDC/NHS, Hydrazine is injected to form hydrazide. Once the surface sample is injected a hydrazide-aldehyde bond is formed rapidly. Further stabilization can be reached with cyanoborohydride.

A detailed protocol can also be found here: https://www.sprpages.nl/immobilization/immobilization-procedures/aldehyde
5.1.2 Amino Groups (-NH2)
The amino groups (-NH2) will be used to covalently attach the surface sample to the sensor surface. After the activation of the surface using EDC/NHS the surface sample is injected over the spots to form bonds with its primary amines. The surface will be deactivated/blocked after the surface sample injection. Activated and blocked spots can be used as control surfaces in the following experiments.

A detailed protocol can also be found here: https://www.sprpages.nl/immobilization/immobilization-procedures/amine
Example of a Direct Coupling on Sierra SPR-32
As an example, the immobilization on 16 sensor spots (1-8 C+D) is described in detail, whereas the active surface (activated / coated with target protein) will be sensor spot 1-8 D and the reference surface (activated/blocked) will be sensor spot 1-8 C.
Activation (EDC/NHS):
- Injected for 6 minutes at 10 µL/min
- Injected over Sensor Spot 1-8 C+D
Target Protein:
- Injected for 6 minutes at 10 µL/min
- Injected over Sensor Spot 1-8 D
Blocking (Ethanolamine):
- Injected for 6 minutes at 10 µL/min
- Injected over Sensor Spot 1-8 C+D

5.1.3 Thiol groups (-SH)
One approach is the ligand thiol coupling. Thereby the thiol groups (-SH) will be used to covalently attach the surface sample to the sensor surface. After the activation of the surface using EDC/NHS, PDEA is injected to form a bond with the surface sample to be announced as active. The surface will be deactivated/blocked after the surface sample injection. Activated and blocked spots can be used as control surfaces in the following experiments.
Another approach is the surface thiol coupling. Thereby proteins with a pI below pH 3 can be immobilized by a similar approach even though they have no free thiols. The alternative procedure of surface thiol coupling requires to modify the ligand “outside” the SPR machines with a thiol-reactive moiety like PDEA. The sensor surface is modified to have terminal free thiol groups (e.g. from cystamine) and the modified ligand is then injected onto the sensor surface similarly to the ligand thiol coupling.
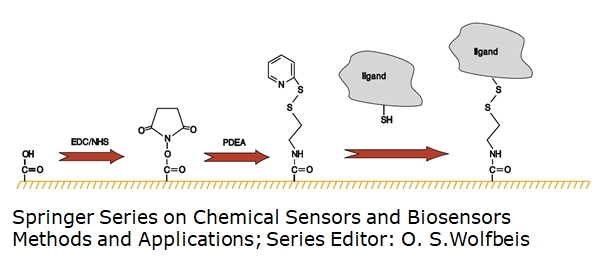
A detailed protocol can also be found here: https://www.sprpages.nl/immobilization/immobilization-procedures/thiol
5.2 Indirect coupling / capture
5.2.1 Antibody based capture systems
In the case of indirect coupling / capture methods the surface sample is bound to a second molecule, which is covalently coupled to the sensor surface. In most cases the captured surface sample can be regenerated, thus a reuse of the surface is possible.
The most common system is an anti-XY antibody, as described in table 5-1.
Table 5-1: Common antibody capture molecules
| Capture molecule | Supplier / Product code | Regeneration |
|---|---|---|
| Goat-Anti-Human | Jackson Immuno Research, Code: 109-005-008, 2mg | 1 min. injection, 10 mM Glycine, pH 1.5 |
| Rabbit-Anti-Mouse | Jackson Immuno Research, Code: 315-005-008, 2mg | 1 min. injection, 0.1 M HCl |
| Anti-Flag-Tag | Sigma Aldrich, Code: F3165-, 0.2mg | 2×1 min. injection, 10 mM Glycine, pH 2.0 |
| Anti-His | Qiagen 34660 | 2×1 min. injection, 10 mM Glycine, pH 2.0 |
5.2.2 His-Tag Capture Systems (Bruker Product: 1862617 / 618)
The sensors contains a dextran matrix with pre-immobilized amine-NTA and allows the capture of His-tagged surface samples (His6 or His10). After an activation with e.g. Ni 2+ , the surface sample can be injected. The reusability of the sensor is given due to its regenerability using EDTA. Further details can be found here: https://www.sprpages.nl/sensor-chips-intro/biacore-sensor-chips/nta
5.2.3 Biotin-Tag Capture Systems (Bruker Product: 1862620 / 621)
The sensor contains a dextran matrix with pre-immobilized Neutravidin and allows the capture of biotinylated surface samples. The interaction between Biotin and Neutravidin is amongst the strongest known . Further information can be found here: https://www.sprpages.nl/sensor-chips-intro/biacore-sensor-chips/sa
5.2.4 IgG Capture (Bruker Product: 1862623 / 624)
The sensor contains a dextran matrix with pre-immobilized Protein A/G and allows the capture of most subclasses of e.g. human, mouse, rat, horse IgG. The surface is regenerable with e.g. 10 mM Glycine, pH 1.5-2.5.
5.2.5 Self-made capture surfaces
In order to create your own capture surface the following items could be ordered and directly immobilized to a HCA (#1862614 / 1862615) or Amine sensor (#1862611 / 1862612).
Table 5-2: Overview of molecules to create self-made capture surfaces
| Capture molecule | Supplier / Product code | Regeneration |
|---|---|---|
| amine-NTA | Sigma-Aldrich, Product#: 14580, 5G | 50-100 mM EDTA, pH 8 |
| Neutravidin | Thermo Fischer, Product#: 31000, 10 mg | – |
| Protein A / G | Thermo Fischer, Product#: 21186, 5 mg | 10 mM Glycine, pH 1.5-2.5 |
5.3 Direct vs. indirect
In order to decide which immobilization method to use the following advantages and disadvantages from SensorCon-2 could be used.

5.4 Immobilization levels
The binding responses of the injected solution sample depends on immobilization / capture levels of the surface sample, expecting there is binding / interaction at all. In order to calculate an application depending maximum response level use equation (5-1) [https://www.sprpages.nl/sensorgram-tutorial/a-curve].
| (5-1) |
Due to lower activity of the immobilized / captured surface sample the calculated R max is often higher than the experimental R max . The shown equation can also be used to calculate back the RLigand, or immobilization / capture level of the surface sample.